Thermochemical nitriding processes
There are three common nitriding processes. The chosen method depends on the specific application of the final nitrided components.
The nitriding processes are described briefly below. To get a detailed description of the processes and how each one affects the properties of the nitrided components or parts,
download the full application note.
Salt bath nitrocarburizing
After preheating, the components are submerged into a salt bath consisting of alkaline cyanate and alkaline carbonate. Through oxidation and thermal reaction, the alkaline cyanate releases nitrogen and carbon, which diffuse into the surface of the metal.
After salt bath nitrocarburizing, the component is quenched in an oxidizing salt bath. This produces a black iron oxide (Fe
3O
4) that fills the pores of the compound layer and provides additional corrosion protection.
- Typical applications: Parts for the automotive industry, such as piston rods, camshafts and gears, as well as parts used in the aircraft, offshore and mechanical engineering industries.
Gas nitriding and gas nitrocarburizing
In gas nitriding, the component is placed in a sealed, bell-type nitriding furnace. When nitriding temperature is reached, ammonia is let into the furnace. As the ammonia reacts with the metal, it decomposes and releases nascent nitrogen, which diffuses into the surface of the metal. In gas nitrocarburizing, carbon is added to the gas.
- Typical applications: Machine spindles, ductile iron pump housings, door lock mechanisms, water pump components and pistons for gas compressors.
Plasma nitriding and plasma nitrocarburizing
Plasma nitriding is carried out in a nitrogen/hydrogen atmosphere. The plasma is produced in a vacuum chamber with a high voltage. In this environment, the metal component acts as a cathode and the vacuum vessel as an anode. The plasma nitrocarburizing process is the same, but gases containing carbon are added.
- Typical applications: As plasma nitriding allows for a large variety of nitride layers, the components can be used in many different applications. These include camshafts and crankshafts in high performance motors, machine spindles, autobody blanking dies, corrosion-resistant engine valves and high-speed steel cutting tools.
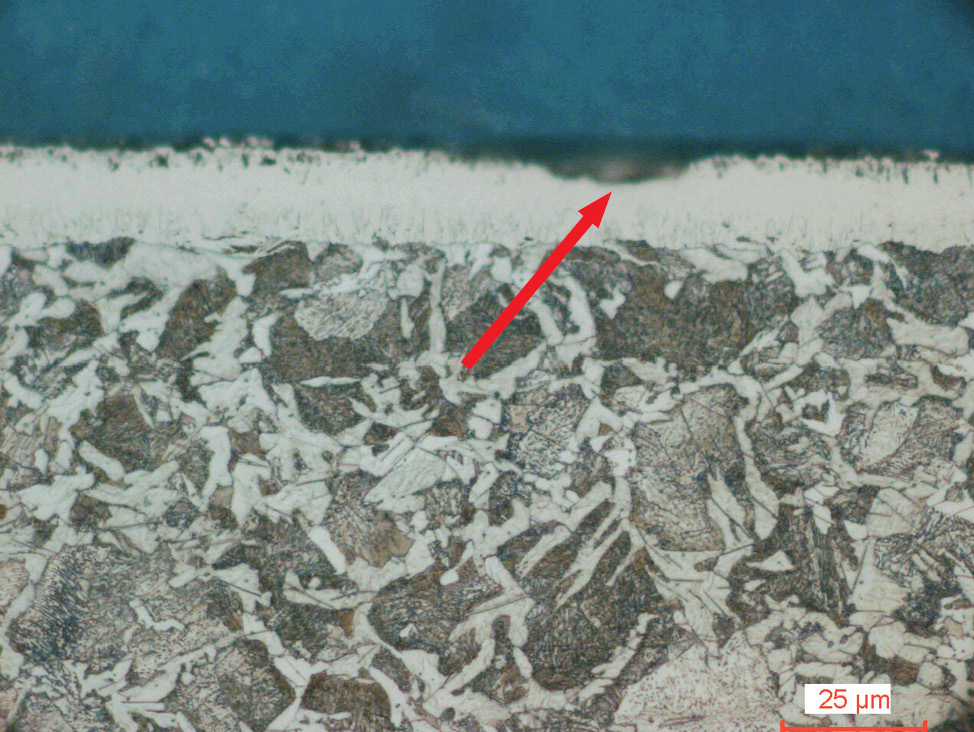
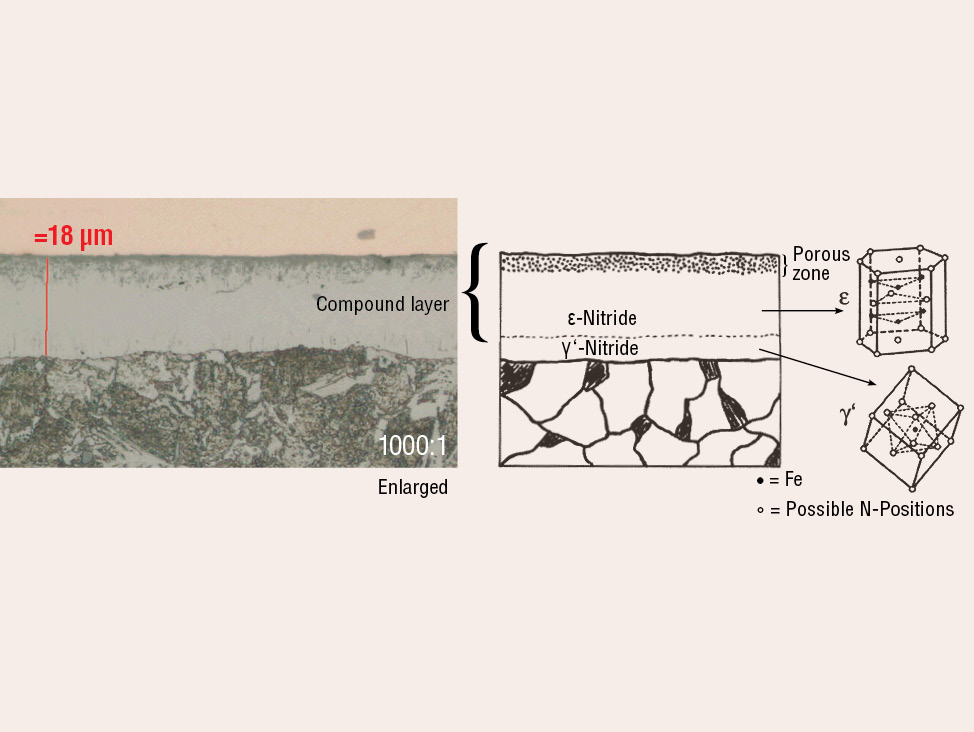
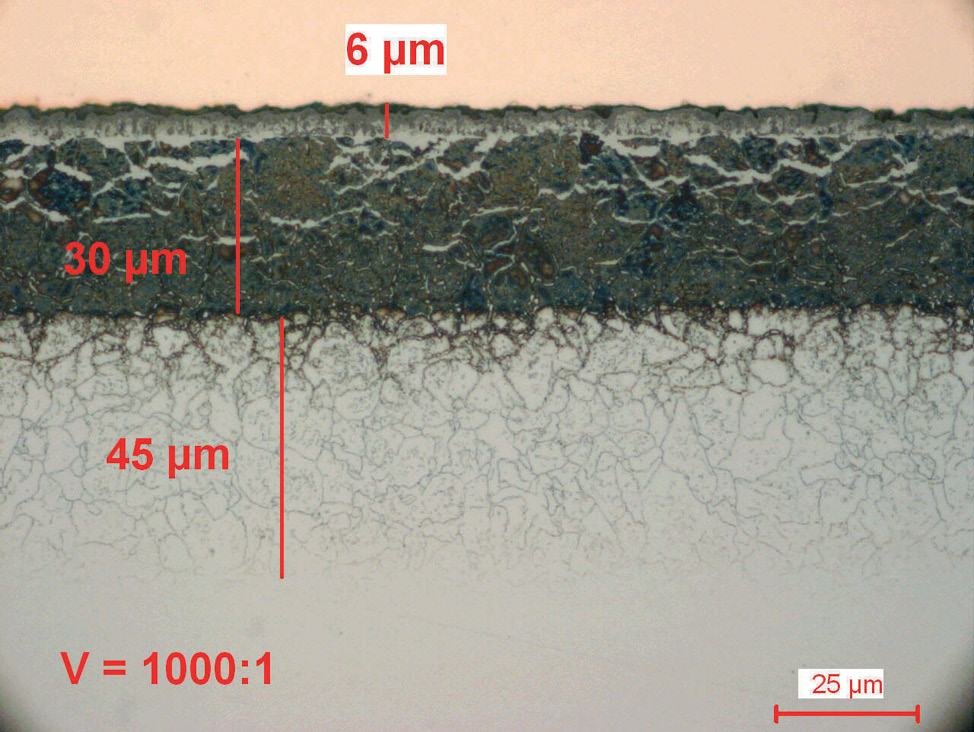
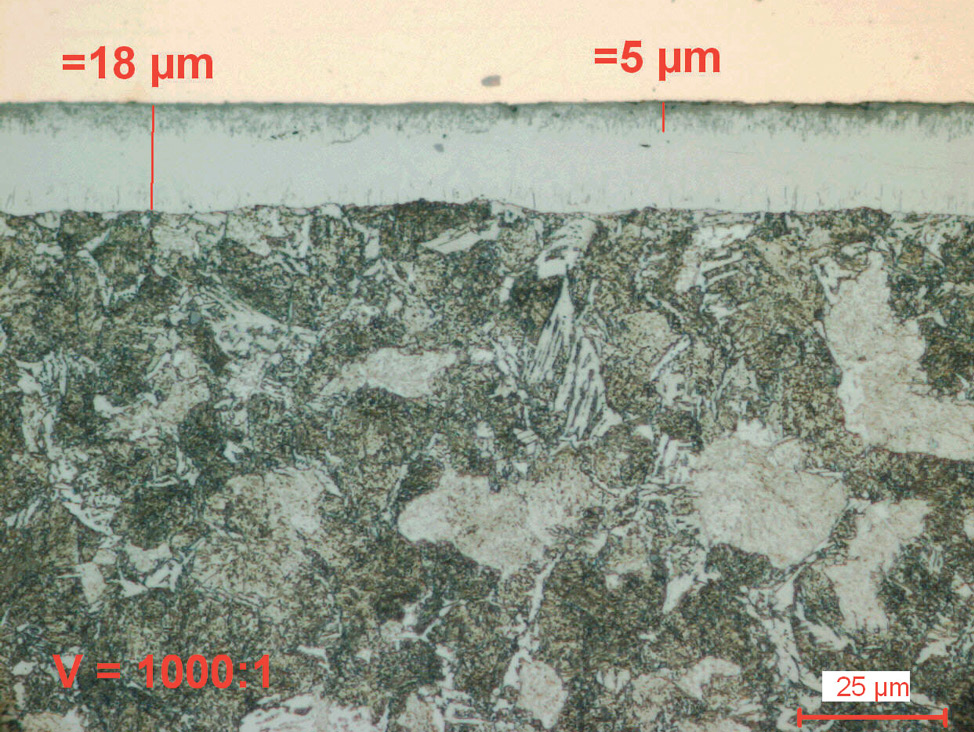 Fig. 4: Salt bath nitrocarburized steel alloy (16MnCr5), etched with 1% Nital. The diffusion zone is etched dark and the compound layer with porous zone shows white.
Fig. 4: Salt bath nitrocarburized steel alloy (16MnCr5), etched with 1% Nital. The diffusion zone is etched dark and the compound layer with porous zone shows white.
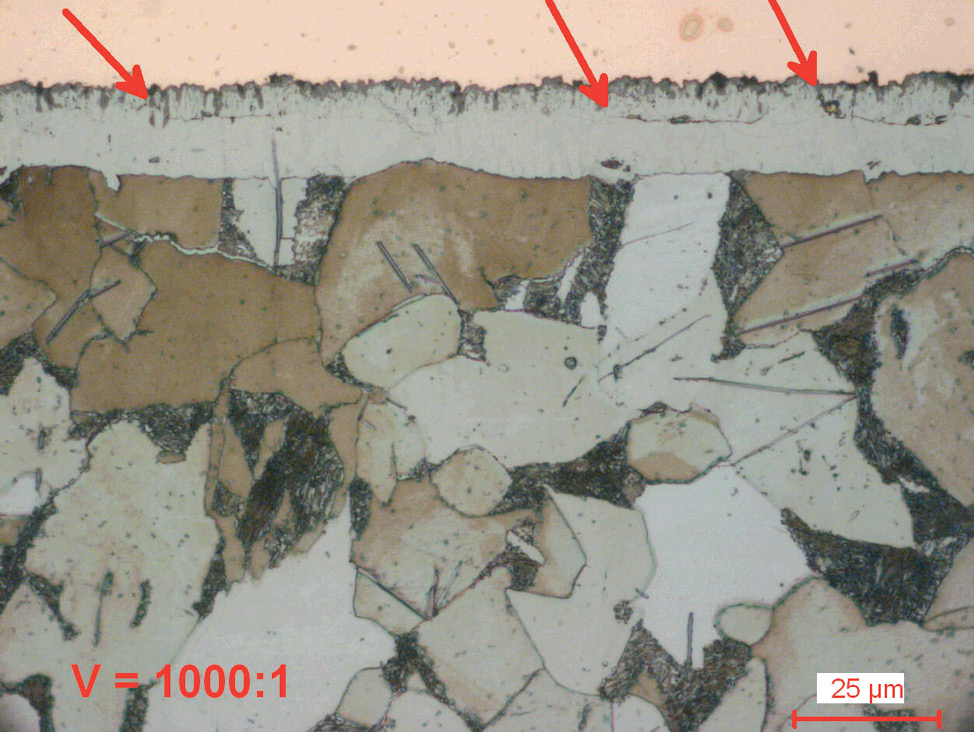
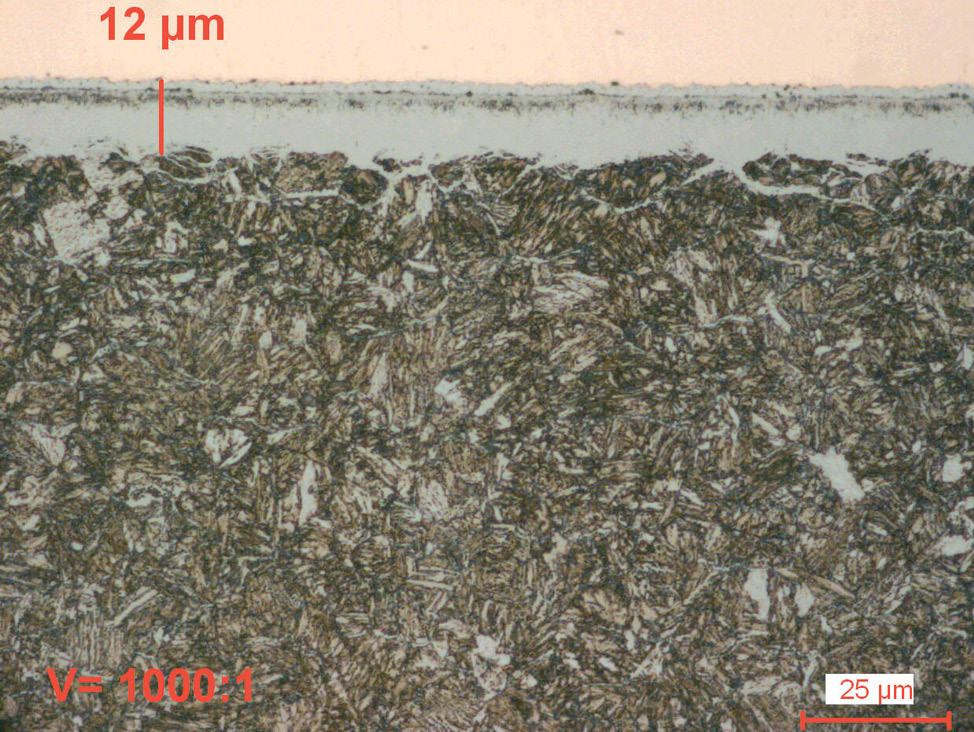
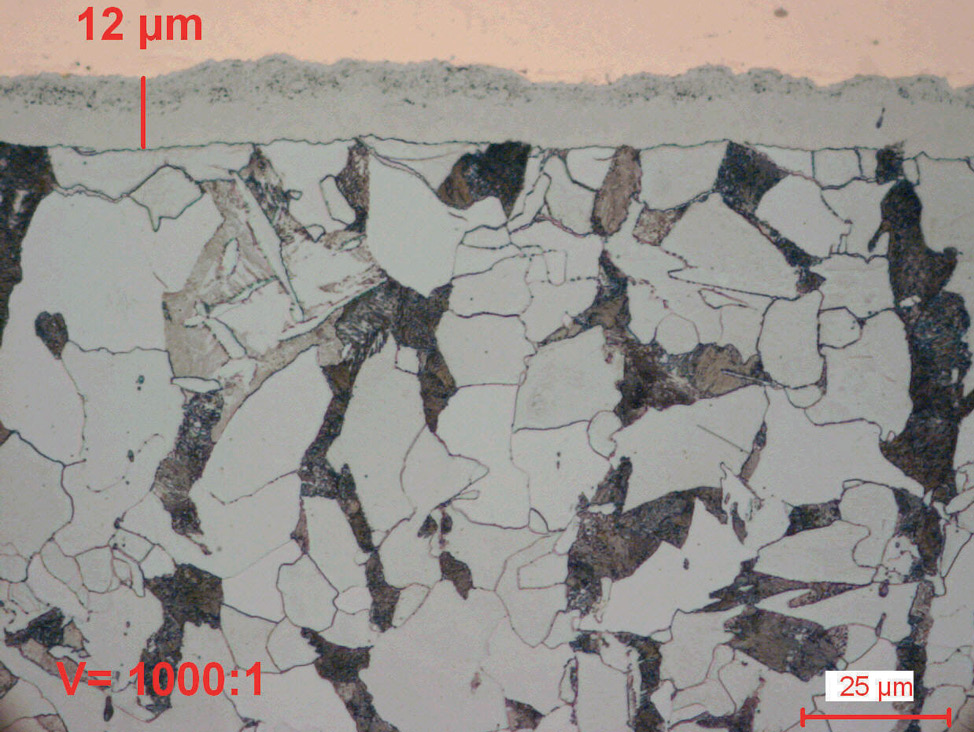 Fig. 5: Gas nitrocarburized carbon steel (580°C for 1.5 hours).
Fig. 5: Gas nitrocarburized carbon steel (580°C for 1.5 hours).
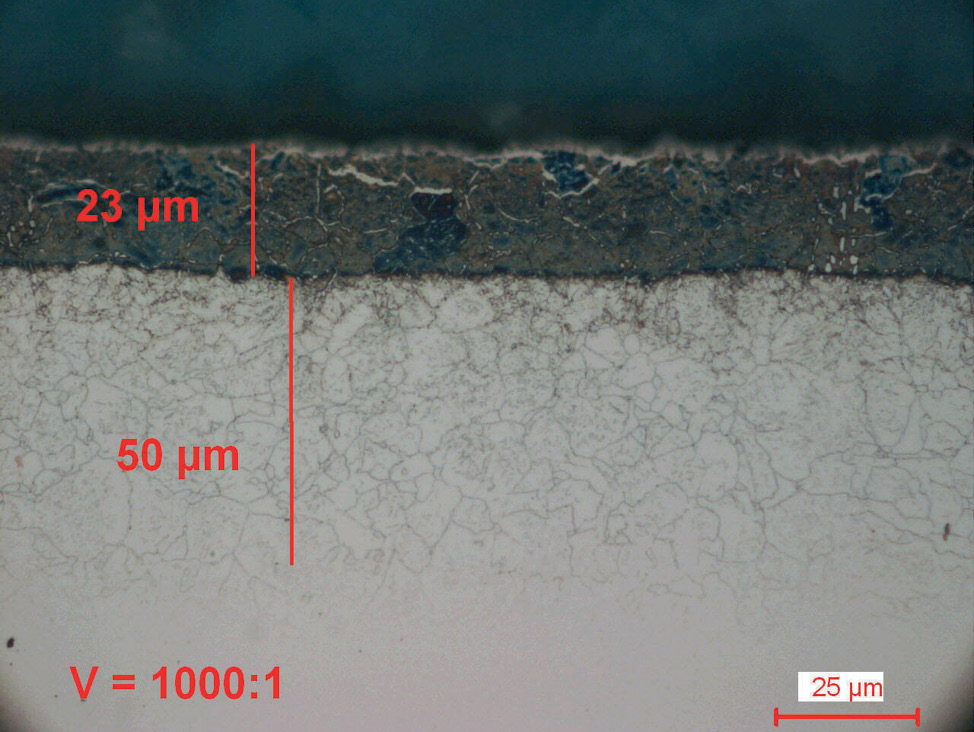
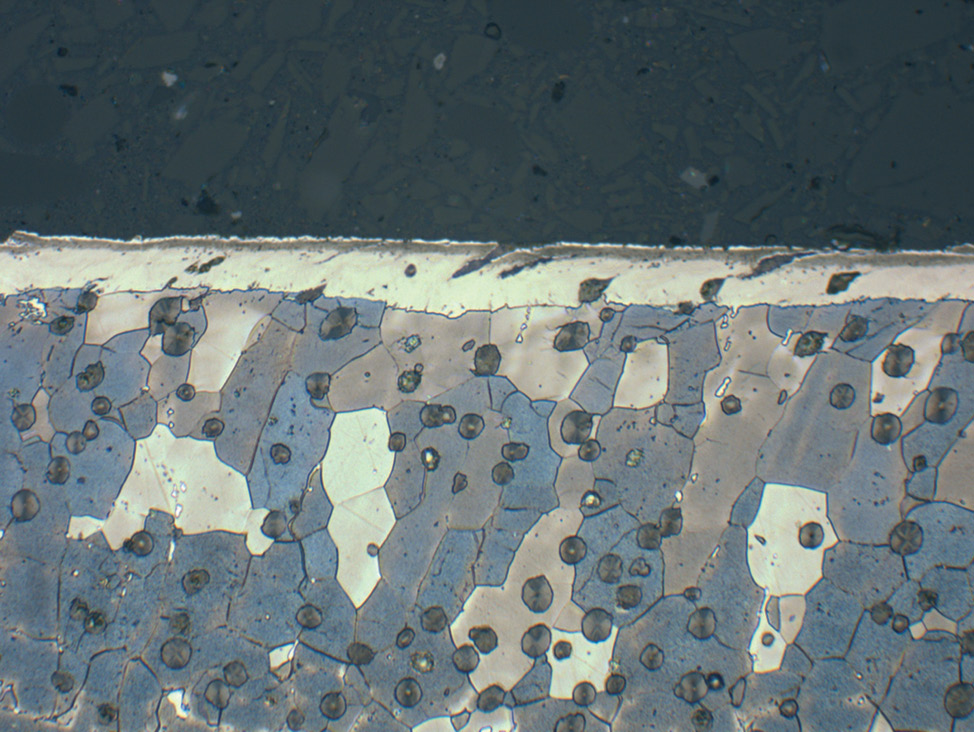
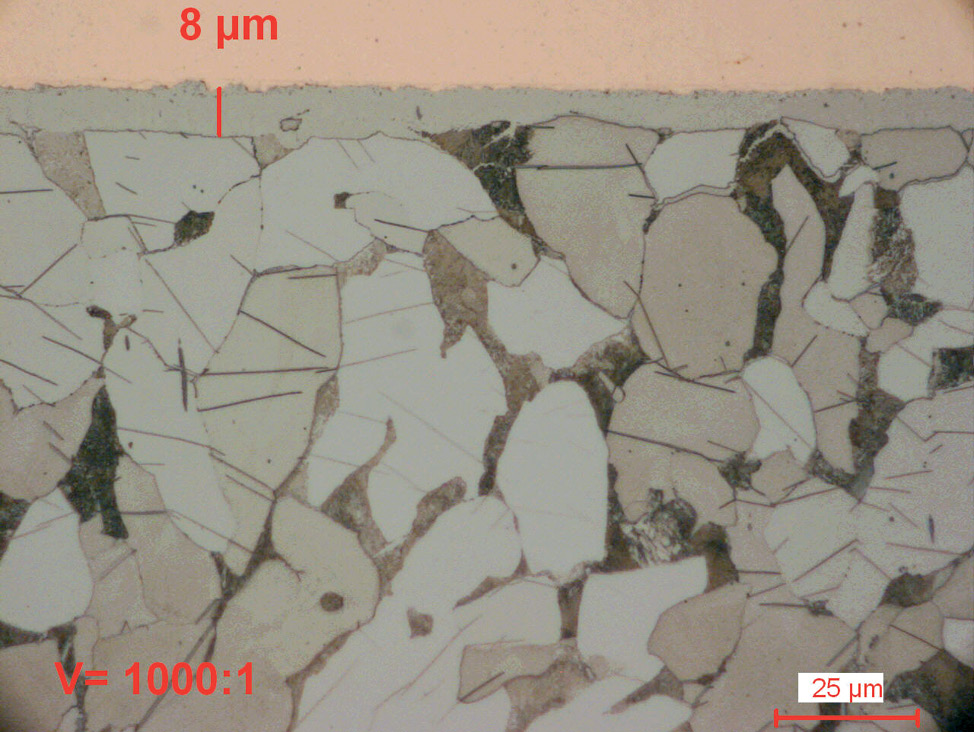 Fig. 6: Plasma nitrocarburized carbon steel (570°C for 6 hours). Both nitride layers are without a porous zone and have a very fine surface finish.
Fig. 6: Plasma nitrocarburized carbon steel (570°C for 6 hours). Both nitride layers are without a porous zone and have a very fine surface finish.